Feasibility Assessment of Potential Standards
Feasibility assessment is the systematic analysis and thoughtful evaluation of a potential standard’s benefits to the field as well as anticipated impediments to its scientific maturity, projected economic impact, and other relevant factors.
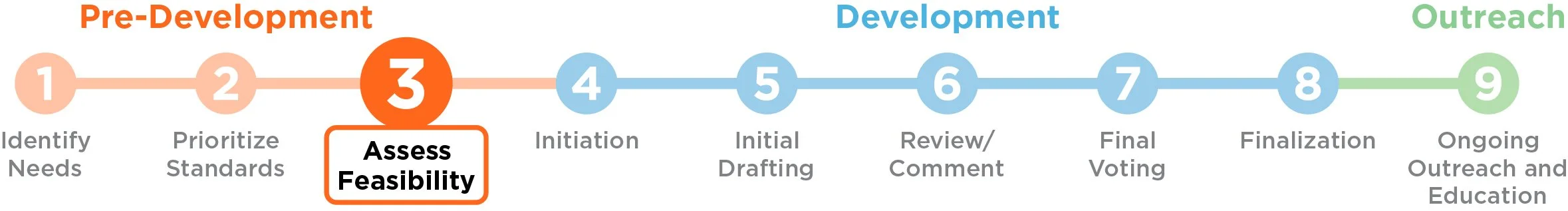
Feasibility assessment is the third step in the standards advancement process. Once the regenerative medicine community has identified and prioritized an area of standards need, it is critical to assess whether there are significant barriers that could impede its development as a standard.
Why Assess Feasibility
Assessing feasibility before moving a standard topic forward for SDO consideration:
Focuses the time and energy of the regenerative medicine community on the standards with the best chance of moving successfully through the development process
Avoids wasting resources on standards that may become stalled during development
Learn more about the vital role feasibility assessments play in standards development.
Feasibility Assessment Approach
On a semi-annual basis, SCB organizes feasibility assessment meetings for a selection of high-priority standards identified by the community in the Regenerative Medicine Standards Portal. These meetings bring together regenerative medicine stakeholders with diverse expertise and viewpoints to consider feasibility factors such as:
Technical feasibility: Whether an adequate technical and scientific foundation exists for constructing the standard
Implementation feasibility: Factors that influence an individual firm’s adoption of the standard such as incurred costs; the standard’s compatibility with existing equipment, materials, and technology; and required in-house expertise
Expert availability: Level of interest from technical experts in the field who can advance the standard
The results inform SCB’s standards priorities and often spur the creation of new working groups for any standards or pre-standards outputs selected to move forward.
Feasibility Assessment Reports
Ready for Standards Development
SCB has prioritized these topics for advancement based on favorable feasibility assessments.
Biological Evaluation of Tissues and Extracellular Matrices for In Vivo Studies (pdf)
Evaluating Outcomes of iPSC Differentiation Using ddPCR Quantification (pdf)
Evaluating Pre-existing Immunity to Adeno-associated Viruses
Interoperability Guidelines for Regenerative Medicine Equipment and Software (pdf)
Regenerative Medicine Manufacturing Equipment and Facility Requirements
Not Yet Ready for Standards Development
For topics not ready for formal standards development, SCB often organizes the development of pre-standards outputs (e.g., white papers) that can help the field build consensus and move toward readiness for standardization.
Get Involved
Contact SCB if you would like to be notified of upcoming opportunities to participate in a feasibility assessment.